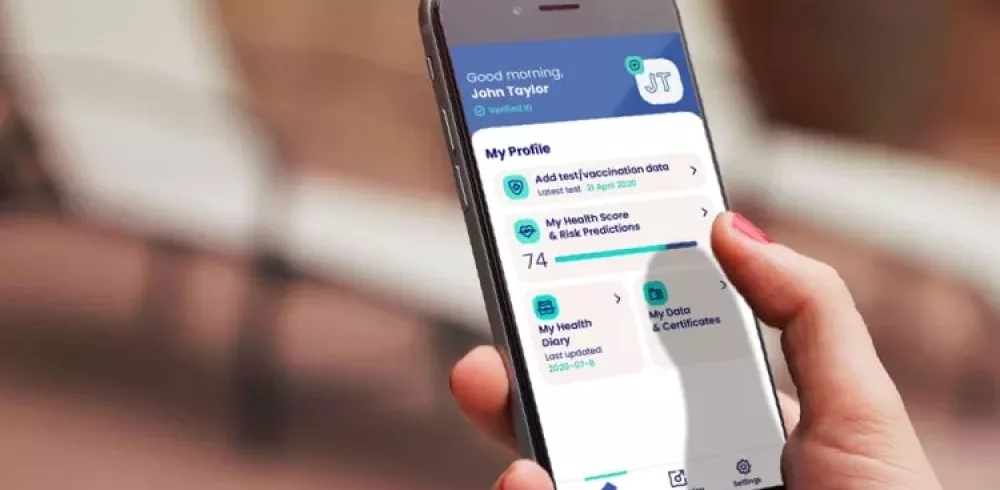
AI Tool Offers Lifeline to Manufacturers Under Threat of Covid-19 Outbreaks : An AI employee health data management tool is offering a lifeline to manufacturers which are under a persistent threat of Covid-19 outbreaks. Created by Delfin Health and DocHQ, Klarity can help manufacturers stay operational during a potential second nationwide lockdown by predicting, monitoring and testing the health and safety of their workforce. It can give them a real-time clinical understanding of the health of their workers which include assemblers, welders, machinists, production managers and quality control inspectors.
“The very nature of manufacturing means there are many different functions and roles that typically work in close proximity of each other,” says Will Cooper, Founder and CEO at Delfin Health. “We’ve seen large numbers of workers testing positive for Covid-19 at numerous factories, with some having no option but to suspend production as a precaution.
“If manufacturers are to remain open, they need to monitor the health and safety of their staff in the most efficient way. They need to enforce procedures such as social distancing, splitting teams and finding ways to remove direct contact. Introducing daily symptom checking and risk assessment is also critical, which is where tools like Klarity can help.”
The comprehensive solution will enable manufacturers to address their employees’ anxieties associated with returning to a safe work environment and about the future. It offers health risk assessments using explainable AI to determine the severity of Covid-19 for each employee. It also offers accurate and ongoing testing to assess their Covid-19 status, and continuous surveillance and symptom checking to inform and reassure employees.
The various testing methodologies, which include group and randomised testing, allow employers to reduce the amount of testing required and minimise the risk of an outbreak in the currently active workforce, in particular by identifying asymptomatic cases which are thought to play a significant role in the transmission of the disease.
The testing process, based on World Health Organisation (WHO) protocols, is guided by healthcare professionals who oversee and validate the results and use accredited tests including Polymerase Chain Reaction (PCR), Enzyme-Linked Immunosorbent Assay (ELISA), Saliva (MHRA approved) and Rapid RT-LAMP and where relevant, rapid antibody tests. Further follow-up consultations can be provided as required.
Will adds: “It’s not enough for employers to simply rely on people using the test and trace government solution which tests only symptomatic people. There are cases of the virus spreading rapidly throughout manufacturing plants due to the conditions of the facilities which typically involve close contact. It’s also highly likely these plants relied on just testing or sending home symptomatic people. It requires a systematic process of regular testing.
“While regular testing might appear to be cost prohibitive, manufacturers using our technology can reduce costs by between 50-75% while also limiting the risk of asymptomatic spread of the virus.”
Manufacturing & Engineering Magazine | The Home of Manufacturing Industry News