Nowadays, 3D printing is used for different patient related treatments in the medical industry, with one of the key areas being the development of models for planning and preparation aid in surgical work. 3D printing tools have been used so far to produce metallic parts for structural purposes in orthopaedic, spinal, and craniomaxillofacial areas.
However, several studies have proven that not all patients can tolerate metallic based implants, which led to a research on non-metallic materials. Mineral based ceramics and certain class of polymeric materials are biocompatible and without proven immunological reactions.
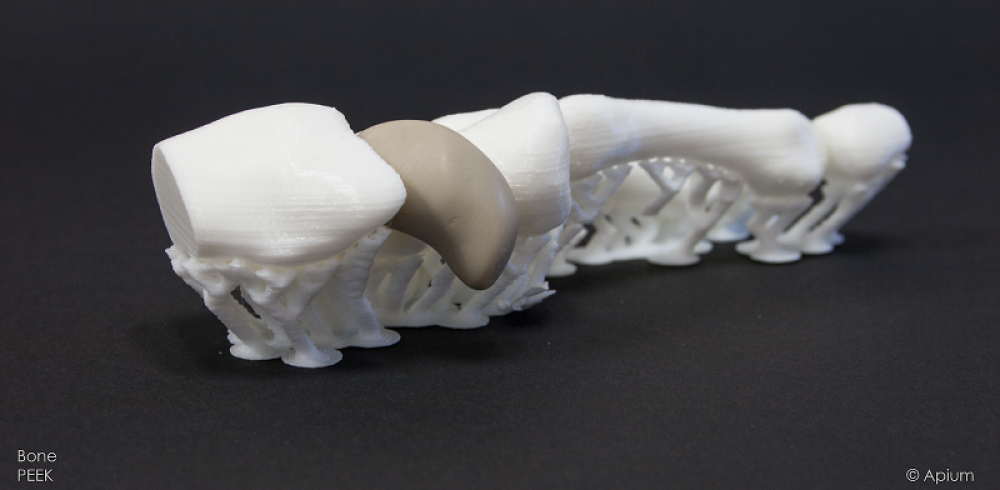
PEEK (Polyetheretherketone) is one of these materials and it is known to exhibit outstanding mechanical and biocompatible properties. It has a modulus which is closer to human bone than any metallic implant material and thus is preferred in medical bone implant applications where stress-shielding is a potential biomechanical challenge. Due to its relatively lower load-bearing capacity relative to metallic materials, PEEK has gained wider usage in the craniomaxillofacial and spine implant based treatments.
Until recently, PEEK could not be fabricated using additive manufacturing (AM) tools like 3D Printing because it was an expensive, complicated approach that changed PEEKâs properties at a molecular level. However, Apium Additive Technologies GmbH has demonstrated that PEEK material can be 3D printed using the Material Extrusion method. The M220 machines have been conceived to preserve biocompatibility and sterile properties of processed PEEK filament, allowing Apium fulfil its promise of providing a tool capable of revolutionizing patient care.
The 3D printed PEEK implants use data from CT or MRI imaging of patients and generate 3D Printer readable data formats after the raw imaging data has been analysed, segmented and reconstructed using slicing software tools. This finding has been argued to lead to better treatment of medical trauma cases in CMF, hand and spine surgery.