Sciad Communications / Watson-Marlow Fluid Technology Solutions (WMFTS) will showcase its peristaltic pumps and fluid path technologies for the chemistry and life science industries during Ilmac in Basel, Switzerland (16 – 18 September 2025).
Visitors to Stand B196 in Hall 1.0 can learn from WMFTS life science experts on how the company can support processes throughout upstream, downstream and final fill/finish applications with its scalable peristaltic cased pump range, specialised filling machines and single-use assemblies.
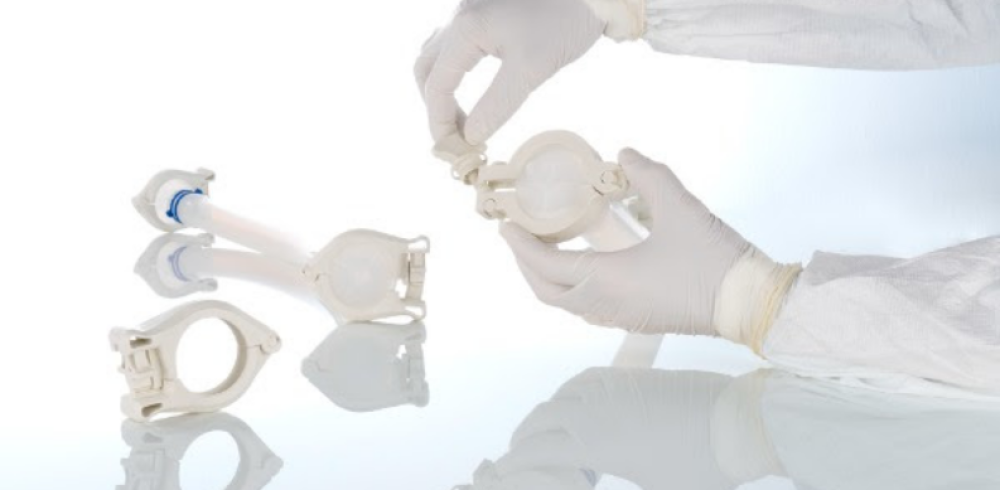
The focus will be on single-use fluid-path solutions from the BioPure and WMArchitect™ brands. WMArchitect™ single-use solutions reduces risk, ensures quality and improves delivery times for life science companies. The hygienic single-use clamp connector from BioPure, BioClamp®, demonstrates how single-use and sustainability can go hand in hand: compared with its predecessor, BioClamp is 13% lighter and reduces the lifecycle carbon footprint by 26%.
Also on display will be WMFTS’s range of specialised peristaltic pumps and critical fluid path technologies for scalable drug bioprocess and small-batch drug product fill/finish applications.
Rolf Bächli, Sales Engineer, Life Science OEM / Medical Devices and Diagnostics Sector at WMFTS, said: “As well as discovering what our expanding portfolio of products is capable of, visitors to Ilmac in Basel will be able to discuss their unique fluid management challenges with our life science experts. Our team will be able to provide advice on how to ensure compliance, process stability and final product quality.
“Our OEM panel mount pumps deliver ultra-precise flow with quiet, efficient performance. With closed-loop control, Industrial Ethernet, and plug-and-play integration via DriveSure, you get faster installs, less risk, and more time to innovate.”
On display at stand B196:
- WMArchitect™ single-use solutions is an end-to-end biopharmaceutical fluid management solution from a single supplier. WMArchitect single-use solutions is designed to offer both standard, ready-to-use single-use assemblies as well as customised designs that are fully supported with tailored validation packages. WMArchitect will streamline a process, safeguard product, reduce regulatory burden and resolve challenges for end-to-end, single-use fluid path management.
- Watson-Marlow peristaltic cased pumps are globally recognised and validated for qualified bioprocessing duties. For the user, an enhanced operator and control system interface contributes to step-change improvements in validated process security. Not only do Watson-Marlow pumps deliver accuracy of up to +/-0.5% to guarantee process stability and product quality, but closed fluid path pumping assures complete isolation from any source of contamination during transfer, filling and filtration applications. Watson-Marlow pumps are ideal for processes from research to production, with flow rates from 0.004 ml/min to 3.5 L/min and pressures up to 7 bar.
- DriveSure® is a complete peristaltic panel mount pump solution helps OEMs to reduce time-to-market and achieve competitive gain. Visit the WMFTS stand to discover how the cool, quiet and compact design with precise performance makes DriveSure suitable for the demands of laboratories, cleanrooms and process environments.
- Flexicon’s aseptic vial fill/finish systems for biopharmaceutical applications offer class-leading precision, efficiency and flexibility. From stand-alone units for hand filling, through to semi-automatic and fully automatic systems Flexicon offers customised solutions to fit any glass vial, plastic bottle, test tube, eye-dropper or non-self standing microtube. The low shear, gentle pumping action of our peristaltic fillers ensure your valuable product is transferred undamaged with high accuracy and precision.
- Manufactured in ISO 14644-1 class 7 cleanrooms and rigorously tested, Watson-Marlow Tubing is available in over 40 sizes that carry a USP Class VI and FDA ratings, with the latest in non-contact continuous measurement systems that ensures quality of every batch.
- BioPure provides a range of single-use solutions to simplify production operations, lower cGMP manufacturing costs and reduce process validation.
Also at Ilmac 2025, Calum Love, Sustainability Technical Lead at WMFTS, and Marco Renggli, Sales Engineer Pharma and Biopharma End-Users at WMFTS, will feature in a discussion on the transformative power of lifecycle thinking in product redesign for lower environmental impact.
The session on September 16, (1:20pm to 1:50pm; Europe/Zurich) at the Future of Life Sciences Area, aims to inspire, inform, and equip you with the knowledge to begin reducing your product’s carbon emissions.
This session will reveal how a simple plastic clamp became a case study in using life cycle assessment and eco-design to reduce its carbon footprint by 26% through design and manufacturing improvements, supported by data-driven decision-making.
Manufacturing & Engineering Magazine | The Home of Manufacturing Industry News